How to lower the acidity of the soil
As is known, soil acidity is indicated by the symbols pH and is a measure of the activity of hydrogen ions (H+) in the soil solution. This indicator affects many parameters such as: nutrient availability, fertiliser efficiency, activity and diversity of microbial populations as well as the activity of certain pesticides. Soil pH is determined to provide information on soil chemical properties.
Soil pH is defined as: pH = – lg [H+]
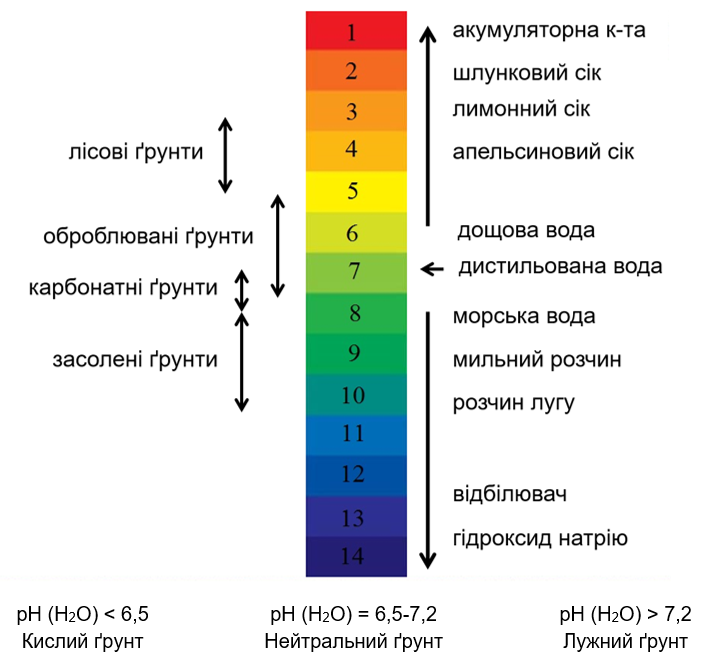
Soil pH is usually measured in a soil/water solution (aqueous pH), but there are other ways of measuring it, such as salt pH (0.01 M CaCl2). The presence of soluble salts in the soil affects the pH value. For most crops, the optimum pH range (H2O) is 6.0-7.5. The range of pH values is 0-14:
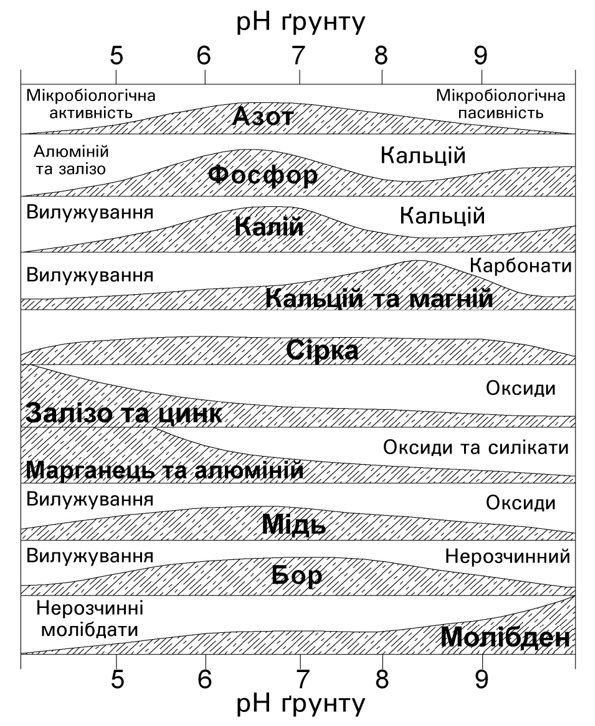
As mentioned above, the pH influences the availability of nutrients. Most nutrients are available between 5.5 and 7.0:
Soil pH can change from time to time (during or between years) as the amount of soluble salts in the soil constantly varies depending on factors such as: rainfall, type of fertiliser, soil bacteria activity and salt content of irrigation water.
Acidic and highly acidic soils require acidity reduction. Note that acidity reduction is the process of bringing the pH to an optimum value (~7.0). In the case of acidic soils, buffer pH (pH buffer) measurements are carried out to determine:
- the amount of metabolic acidity that needs to be neutralised;
- The need for lime to neutralise excess free hydrogen ions (H+) and raise the pH to an optimum value (~7.0).
Thus, the lime requirement of the soil is calculated according to the soil pH value (water and buffer) and from the neutralising capacity of the limestone material used. It is known that acidic soils with a high clay and organic matter content will require more lime.
Liming materials differ in their neutralising capacity. This value is influenced by two factors. One factor is the calcium carbonate content; the other factor that affects the neutralising power of the lime is the fineness of the grind. Lime (CaCO3: 56.0 % CaO; 44.0 % CO2), dolomite or dolomite flour (CaCO3∙MgCO3: 30.4 % CaO; 21.8 % MgO; 47.8 % CO2) and defecate (sugar production wastes which are produced during the sugar juice production process and contain lime) are the most commonly used materials for lime treatment. But if defecate is used, it must always be checked for lime content and concentration.
The following chemical reactions can schematically represent the lime treatment process:
1. Lime application
CaCO3 + 2H+ → Ca2+ + H2CO3
H2CO3 → CO2↑ + H2O
2. Application of dolomite or dolomite flour
CaCO3∙MgCO3 + 4H+ → Ca2+ + Mg2+ + 2H2CO3
H2CO3 → CO2↑ + H2O
In simple terms, the liming process can be described as follows: When lime (CaCO3) is applied to the soil, it decomposes into a calcium cation (Ca2+) and a carbonate anion (CO32-) in the soil solution. Carbonate anion reacts with hydrogen cation (H+) to form carbonate acid (H2CO3) which is unstable and decomposes to form carbon dioxide (CO2) and water (H2O). That is, when lime is applied in the soil solution, hydrogen (turns into water) is replaced by calcium (remains in the soil). With dolomite flour and defecate a similar process takes place, but it also introduces a magnesium cation (Mg2+) into the soil.
Lime should be applied in the autumn in order to give the lime enough time to neutralise the acidity of the soil. As noted, lime doses to reduce soil acidity are calculated according to the pH value (buffer) and can be quite large. But the whole amount of lime cannot be applied at once; it should be a long-term operation, usually between 2 and 6 years. The amount of lime needed should be proportionally spread over this period of time.
It is also worth bearing in mind the effects that can occur after lime treatment. In the first instance, these can occur in the form of micronutrient deficiencies (zinc, manganese, copper, iron and boron) due to their conversion into an insoluble form. Attention should also be paid to the amount of available phosphate, which can be reduced as a result of calcium fixation of phosphate.
Conclusion:
- The acidity of the soil is reduced by liming, according to calculated doses of lime after measuring pH (H2O) and pH (buffer);
- The following year, after lime treatment, it is advisable to test the soil for pH (H2O) and the amount of available micronutrients (to prevent deficiencies);
- A reduction in soil pH can also be achieved by using fertilisers that do not acidify the soil (e.g. sodium and calcium nitrate).
Write to us
and we will find an opportunity
for cooperation