Acidity of soil (pH)
Soil absorbing complex
The surface of particles of clay, sludge or organic matter carries a negative charge and can attract positive ions (cations) of hydrogen (H+), calcium (Ca2+), magnesium (Mg2+), potassium (K+), sodium (Na+) and others The sum of the smallest colloidal particles in the soil, which determine its ability to contain nutrients (absorbency), is called soil absorbing complex (cationic capacity of soil). These tiny particles of the soil act like a weak acid, causing a sour reaction of the soil (low soil pH). On the contrary, soil particles that contain calcium, magnesium, potassium and sodium cause alkaline reaction (high pH of soil). Calcium, magnesium, potassium and sodium displaced hydrogen ions, reducing acidity. Therefore, alkaline is not only the soil where a lot of lime (calcium), but also saline soils, which have an excess of sodium, although there is little calcium. If the soil is sour, it does not mean that the neutral particles of the soil are in the solution of weak acid. Acidity of most soils is due to the concentration on the surface of small (colloidal) soil particles (humus, clay). It is on the surface of small particles of soil that nutrients are contained and the size of the total surface of these particles depends on the properties of the soil and its fertility.
Soil absorbing complex
The surface of particles of clay, sludge or organic matter carries a negative charge and can attract positive ions (cations) of hydrogen (H+), calcium (Ca2+), magnesium (Mg2+), potassium (K+), sodium (Na+) and others The sum of the smallest colloidal particles in the soil, which determine its ability to contain nutrients (absorbency), is called soil absorbing complex (cationic capacity of soil). These tiny particles of the soil act like a weak acid, causing a sour reaction of the soil (low soil pH). On the contrary, soil particles that contain calcium, magnesium, potassium and sodium cause alkaline reaction (high pH of soil). Calcium, magnesium, potassium and sodium displaced hydrogen ions, reducing acidity. Therefore, alkaline is not only the soil where a lot of lime (calcium), but also saline soils, which have an excess of sodium, although there is little calcium. If the soil is sour, it does not mean that the neutral particles of the soil are in the solution of weak acid. Acidity of most soils is due to the concentration on the surface of small (colloidal) soil particles (humus, clay). It is on the surface of small particles of soil that nutrients are contained and the size of the total surface of these particles depends on the properties of the soil and its fertility.
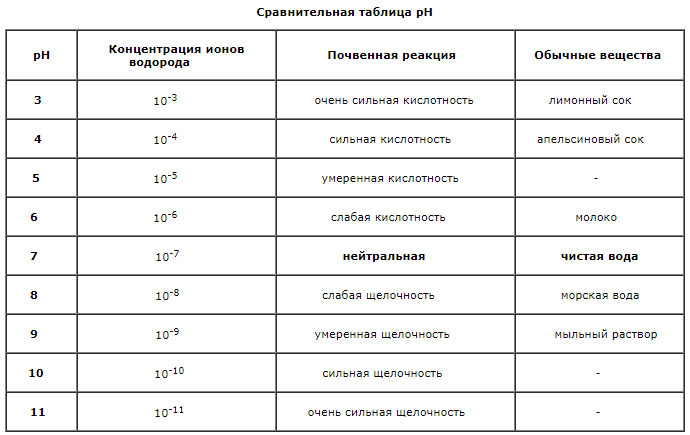
What is pH
Determination of the reaction of the soil is among the most common analyzes. The reaction of the medium (pH) depends on the content of hydrogen ions (H+) and serves as an indicator of acidity or alkalinity of the soil. This index depends mainly on the ion exchange with mineral and organic colloids and the presence of calcium, sodium, potassium and other cations carbonates. Soil medium reaction varies from 3.5 (strongly acidic) – 7 (neutral) to 11 (strongly alkaline). As pH increases, the probability of formation of insoluble hydroxides and carbonates increases. With a decrease in the availability of toxic metals to plants, the pH should be maintained at about 6.5. Acidity of soils is caused by many factors, one of which is the dissociation of functional groups of humus, and the other – the microbiological decomposition of organic matter. Other sources of acidity of soils are clay-silicate minerals and iron and aluminum hydroxide. The intensity of acidification of soils depends to a certain extent on the equilibrium between hydrogen and aluminum ions. With strong acidification of soils, soluble aluminum appears, and the livelihoods of many microorganisms are reduced. Acidity is expressed in terms of pH (decimal point) of the inverse value of the concentration of hydrogen ions (H+), in units of 0 to 14:
pH = – Ig [H+]
The pH value of 7.0 means a neutral reaction, higher alkaline, below acidic. Reducing pH for each unit means increasing the acidity of the soil 10 times.
Cation exchange soil
Continuous formation of hydrogen ions H+ occurs when dissolved in groundwater carbon dioxide (CO2), the formation of carbonic acid. Carbon dioxide is released from the roots of living plants during respiration, as well as during the disintegration of organic matter (organic fertilizers). Hydrogen ions can suppress mineral cations in the soil solution, moreover, calcium, magnesium, potassium and sodium ions are in constant motion between the soil particles, the soil solution and the roots of the plants. Filling of calcium, magnesium, potassium and sodium occurs due to the decay of mineral soil particles and the introduction of organic and mineral fertilizers. The high level of cation exchange is characteristic for clay and organic soils, low – for sand.
Caution
When a large amount of one cation is added, others can be pushed into a ground solution, and washed in deep layers of soil. This can happen when a large amount of unbalanced mineral fertilizer is introduced. This is especially dangerous on light sandy soils, where there are few smallest (colloidal) particles, therefore, the mineral fertilizers are reduced there, broken down into several inputs.
Why the soil is acidified
In general, acidic soils are characteristic of areas where rainfall is quite high. Rain and snow increase the amount of moisture in the soil, and the concentration of calcium and magnesium in the soil solution decreases. Calcium ions and magnesium from the soil particles pass into the soil solution and, in the final analysis, are washed out of the soil. Their place on the soil particles is occupied by hydrogen ions H+, the soil is acidified and re-introduction of lime is required.
Where rainfall is greater than 500 mm / year, significant annual losses of calcium due to washing out occur. Approximately the same amount of calcium is extracted from the soil with high yields. The introduction of mineral fertilizers, such as ammonium sulfate or the use of sulfur, may also oxidize the soil.
Carbon dioxide dissolved in groundwater is a powerful solvent of calcium compounds, translating, in particular, insoluble CaCO3 calcium carbonate into calcium soluble Ca(HCO3)2. With the growth of soil microorganisms, a lot of carbon dioxide is released into the soil, leading to calcium loss because of washing it out of the soil in the form of bicarbonate.
Why soil acidity is important
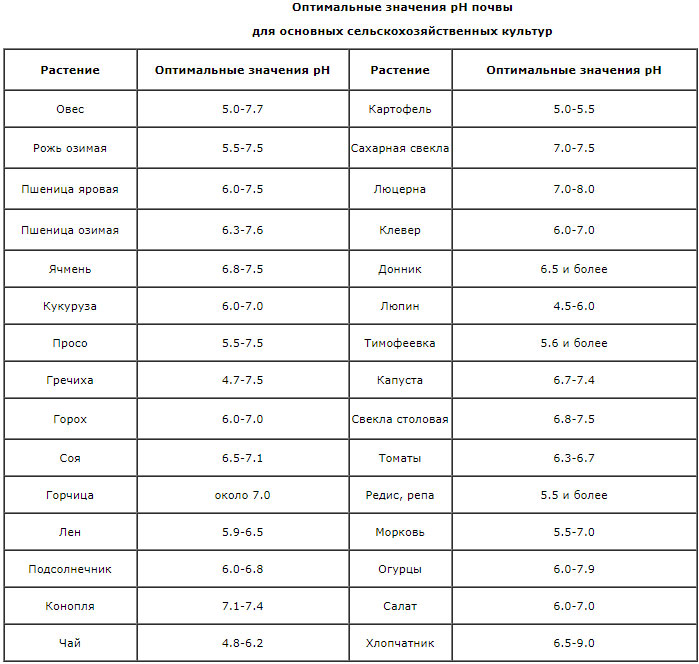
Excessively high (above 9) or low (below 4) the level of acidity of the soil is toxic to the roots of plants. Within these values, pH determines the behavior of individual nutrients, deposition or transformation into forms inaccessible to plants. In acidic soils (pH 4.0-5.5), iron, aluminum and manganese are in forms available to plants, and their concentration reaches a toxic level. In this case, the entry into plants of phosphorus, potassium, sulfur, calcium, magnesium, molybdenum is difficult. On the acidic soil, plant death can be observed without external causes (death from frost, the development of diseases and pests). In protons, in alkaline soils (pH 7.5-8.5) iron, manganese, phosphorus, copper, zinc, boron and most of the trace elements become less available to plants because of the formation of insoluble hydroxides. The optimum is pH = 6.5 (slightly acidic soil reaction). This does not lead to a shortage of phosphorus and trace elements, most of the main nutrients become available to plants, that is, they are in the soil solution. Such soil reaction is favorable for the development of useful soil microorganisms that enrich the soil with nitrogen. Although some species of plants adapted to existence in acidic or, conversely, in alkaline medium, however, most of the plants develop well with neutral or slightly acidic soil reaction (pH range 6.0-7.0).
What is pH
Determination of the reaction of the soil is among the most common analyzes. The reaction of the medium (pH) depends on the content of hydrogen ions (H+) and serves as an indicator of acidity or alkalinity of the soil. This index depends mainly on the ion exchange with mineral and organic colloids and the presence of calcium, sodium, potassium and other cations carbonates. Soil medium reaction varies from 3.5 (strongly acidic) – 7 (neutral) to 11 (strongly alkaline). As pH increases, the probability of formation of insoluble hydroxides and carbonates increases. With a decrease in the availability of toxic metals to plants, the pH should be maintained at about 6.5. Acidity of soils is caused by many factors, one of which is the dissociation of functional groups of humus, and the other – the microbiological decomposition of organic matter. Other sources of acidity of soils are clay-silicate minerals and iron and aluminum hydroxide. The intensity of acidification of soils depends to a certain extent on the equilibrium between hydrogen and aluminum ions. With strong acidification of soils, soluble aluminum appears, and the livelihoods of many microorganisms are reduced. Acidity is expressed in terms of pH (decimal point) of the inverse value of the concentration of hydrogen ions (H+), in units of 0 to 14:
pH = – Ig [H+]
The pH value of 7.0 means a neutral reaction, higher alkaline, below acidic. Reducing pH for each unit means increasing the acidity of the soil 10 times.

Cation exchange soil
Continuous formation of hydrogen ions H+ occurs when dissolved in groundwater carbon dioxide (CO2), the formation of carbonic acid. Carbon dioxide is released from the roots of living plants during respiration, as well as during the disintegration of organic matter (organic fertilizers). Hydrogen ions can suppress mineral cations in the soil solution, moreover, calcium, magnesium, potassium and sodium ions are in constant motion between the soil particles, the soil solution and the roots of the plants. Filling of calcium, magnesium, potassium and sodium occurs due to the decay of mineral soil particles and the introduction of organic and mineral fertilizers. The high level of cation exchange is characteristic for clay and organic soils, low – for sand.
Caution
When a large amount of one cation is added, others can be pushed into a ground solution, and washed in deep layers of soil. This can happen when a large amount of unbalanced mineral fertilizer is introduced. This is especially dangerous on light sandy soils, where there are few smallest (colloidal) particles, therefore, the mineral fertilizers are reduced there, broken down into several inputs.
Why the soil is acidified
In general, acidic soils are characteristic of areas where rainfall is quite high. Rain and snow increase the amount of moisture in the soil, and the concentration of calcium and magnesium in the soil solution decreases. Calcium ions and magnesium from the soil particles pass into the soil solution and, in the final analysis, are washed out of the soil. Their place on the soil particles is occupied by hydrogen ions H+, the soil is acidified and re-introduction of lime is required.
Where rainfall is greater than 500 mm / year, significant annual losses of calcium due to washing out occur. Approximately the same amount of calcium is extracted from the soil with high yields. The introduction of mineral fertilizers, such as ammonium sulfate or the use of sulfur, may also oxidize the soil.
Carbon dioxide dissolved in groundwater is a powerful solvent of calcium compounds, translating, in particular, insoluble CaCO3 calcium carbonate into calcium soluble Ca(HCO3)2. With the growth of soil microorganisms, a lot of carbon dioxide is released into the soil, leading to calcium loss because of washing it out of the soil in the form of bicarbonate.
Why soil acidity is important
Excessively high (above 9) or low (below 4) the level of acidity of the soil is toxic to the roots of plants. Within these values, pH determines the behavior of individual nutrients, deposition or transformation into forms inaccessible to plants. In acidic soils (pH 4.0-5.5), iron, aluminum and manganese are in forms available to plants, and their concentration reaches a toxic level. In this case, the entry into plants of phosphorus, potassium, sulfur, calcium, magnesium, molybdenum is difficult. On the acidic soil, plant death can be observed without external causes (death from frost, the development of diseases and pests). In protons, in alkaline soils (pH 7.5-8.5) iron, manganese, phosphorus, copper, zinc, boron and most of the trace elements become less available to plants because of the formation of insoluble hydroxides. The optimum is pH = 6.5 (slightly acidic soil reaction). This does not lead to a shortage of phosphorus and trace elements, most of the main nutrients become available to plants, that is, they are in the soil solution. Such soil reaction is favorable for the development of useful soil microorganisms that enrich the soil with nitrogen. Although some species of plants adapted to existence in acidic or, conversely, in alkaline medium, however, most of the plants develop well with neutral or slightly acidic soil reaction (pH range 6.0-7.0).

Write to us
and we will find an opportunity
for cooperation