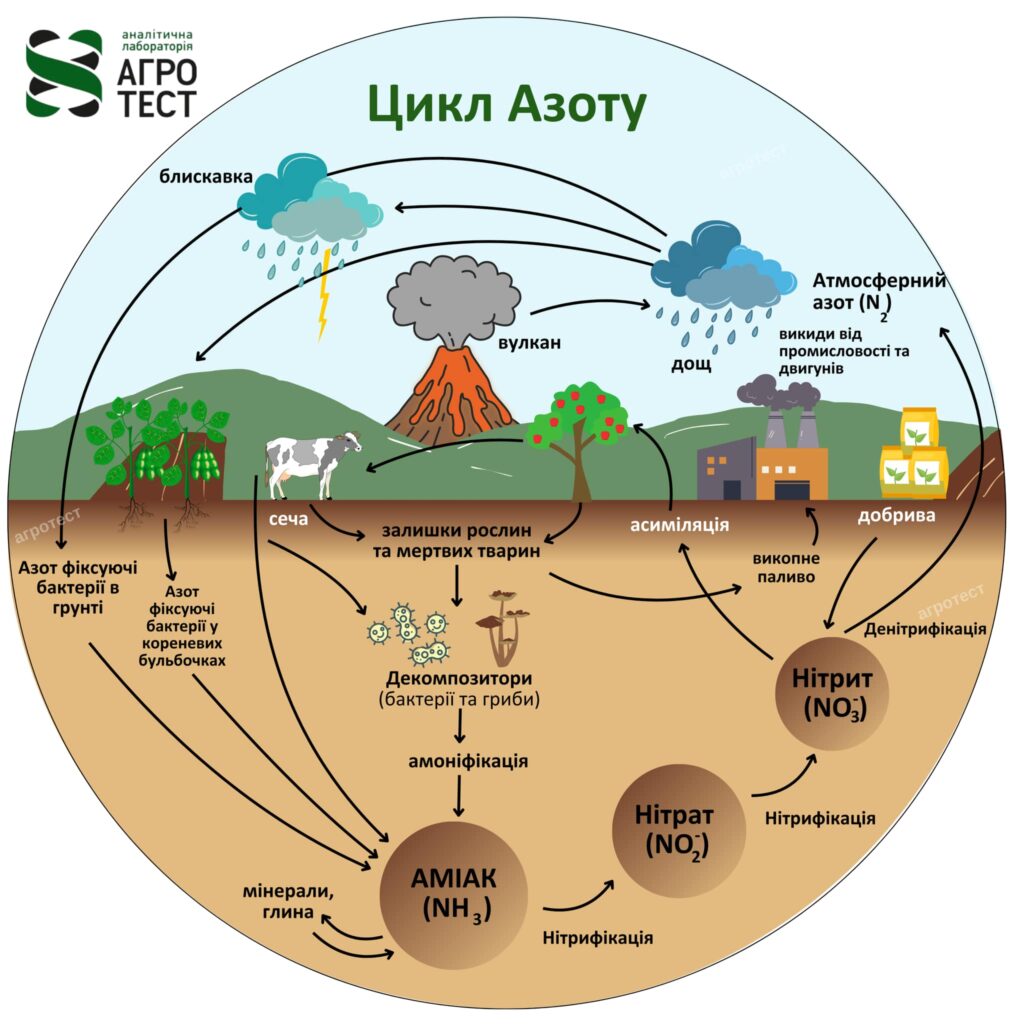
Nitrogen cycle and availability
Where does nitrogen come from?
Nitrogen enters the soil through organic matter such as manure and plant residues. More than 90% of the nitrogen in the soil is in these forms and becomes available only after decomposition by microorganisms.
Nitrogen is also added through fertilizers, which are produced from atmospheric nitrogen (N2) that is converted into ammonia (NH3) for use in fertilizers.
Nitrogen loss – what can hurt your plants?
- Leaching.
The nitrate form of nitrogen (NO3-) is highly soluble and easily leaches out of the soil with excess water.
- Denitrification.
When the soil is saturated with water, some organisms use NO3 for respiration, converting it into gases that are lost from the soil.
- Evaporation
The loss of ammonia (NH3) into the atmosphere from ammonia fertilizers.
- Runoff/erosion
Water that is not absorbed into the soil can carry nitrogen off the field.
How does nitrogen become available to plants?
Nitrogen in the soil goes through several stages of transformation:
- Mineralization – microbes decompose organic nitrogen into ammonium (NH4+), which is absorbed by plants.
- Immobilization – nitrogen is used by microorganisms to form organic compounds, temporarily reducing its availability to plants.
- Nitrification – ammonium is converted into nitrate, which is easily absorbed by plants and is the main form of nitrogen used for their growth.
Always be careful when choosing a fertilizer, as its form significantly affects the period of assimilation by crops. For example, this process takes time, so urea provides a gradual release of nitrogen, which can be useful for long-term plant nutrition. However, it is important to take into account soil and climate conditions to avoid nitrogen losses due to evaporation or leaching.
Write to us
and we will find an opportunity
for cooperation