A practical example of the functions of nature
The energy that all living things (including plants) use for their vital activities is obtained from the sun and assimilated through photosynthesis. Thus, the amount of absorbed and accumulated energy is directly related to the potential life activity of the soil, as part of this energy is released in the form of high-energy sugars that keep the soil microbiology active to perform its functions. Natural energy obtained as a result of photosynthesis stimulates the regenerative system of plants; therefore, our task is to get as much of this energy as possible.
It is known that carbon enters the soil from the atmosphere in the form of carbon dioxide (CO2) and is assimilated as a result of photosynthesis; nitrogen comes in the form of nitrogen gas (N2). The top 20 cm of soil can contain between 5.0 t/ha and 7.5 t/ha of nitrogen gas, which can be made available to plants by nitrogen-fixing bacteria (both legume-associated and free-living). All other unavailable mineral nutrients can also be made available to plants through microbial action. Water enters the soil due to infiltration and is stored in macropores, which is ensured by the stability of aggregates.

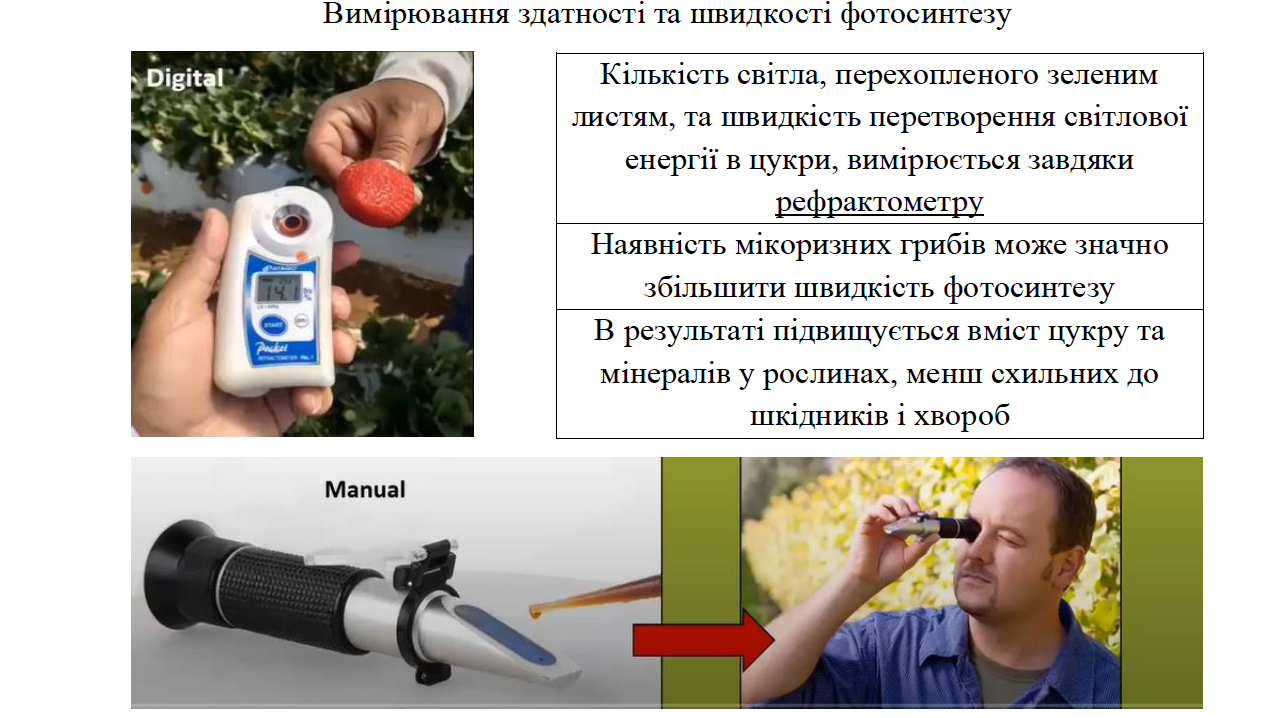
Since photosynthesis is so important, a simple question arises: Is there a way to measure this process? It is believed to be one of the most under-appreciated (measurable) processes in agriculture, as it can provide a wealth of information, including plant health and condition. In general, the rate of photosynthesis can be measured with a simple refractometer (a device that measures the index of refraction of light in a medium); as a material for analysis, squeezed juice from the leaves of agricultural crops is used.
If we consider the main chemical elements found in the soil, most often we are talking about nitrogen. It is important to understand that the bulk of nitrogen in nature is in organic form. If your soil contains 4.0% organic matter, that means you also have about 2.1% organic carbon (because on average soil organic carbon is about 52% of total soil organic matter). The so-called “nature ratio” (first developed by Dr. Cole Albrecht) says the following: the ratio of carbon to nitrogen to phosphorus is 100:10:1. That is, for our example, it will be: 2.1% carbon, 0.21% nitrogen and 0.021% phosphorus. Further decomposition of nitrogen into available forms from unavailable forms occurs thanks to microorganisms and their vital activity by decomposing proteins, peptides and amino acids.
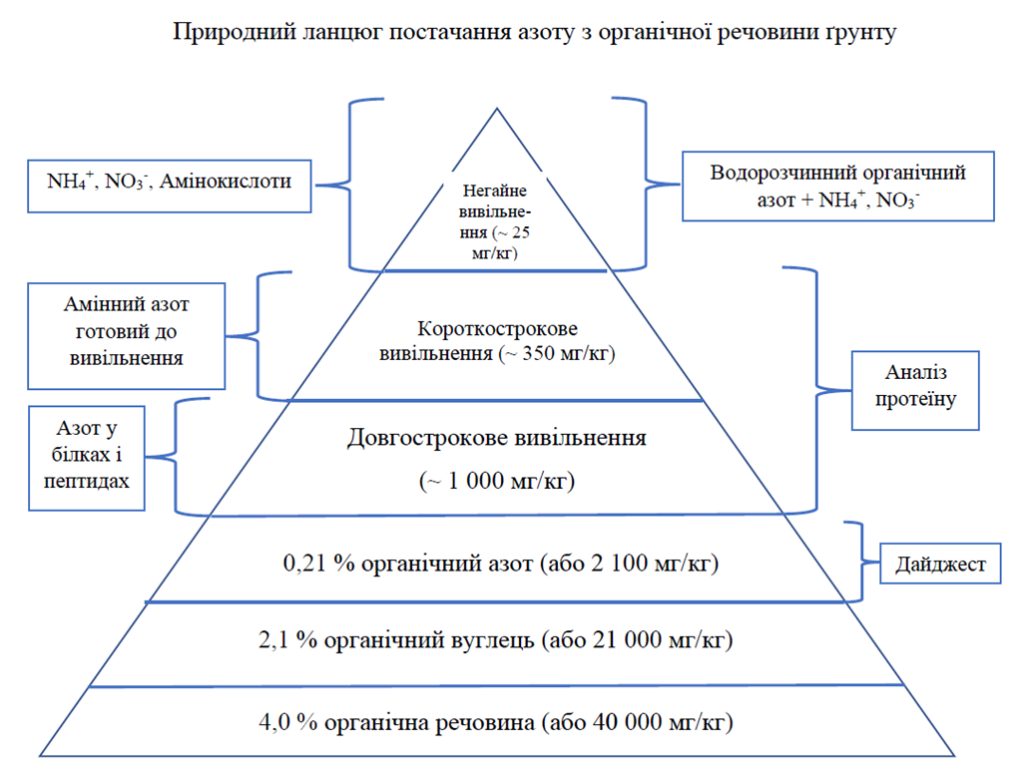
The forms of nitrogen available to plants are water-soluble forms; primarily nitrates and ammonium, and some amino acids. There may be other forms, but these are the most important. In total, there are about 54 different amino acids and amino acid-type compounds identified as available to plants.
There are many methods for determining nitrogen in the soil. One of the most famous is the burning method. This method provides information on total nitrogen (organic + inorganic) and total carbon. Also known is the method with extraction with a solution of potassium chloride, which will give you the total amount of inorganic nitrogen (nitrates + ammonium). Understanding the value of total nitrogen (organic + inorganic), you can subtract from it the value of inorganic nitrogen (nitrates + ammonium); this way you will get the organic nitrogen value.
In the context of the “Soil Health” principles, nitrogen extracted with clean water (water extraction) is currently analyzed. After extraction from the aqueous extract, organic nitrogen, nitrate and ammonium are analyzed; all of these forms of nitrogen are thought to be available to plants because they are water-extractable (dissolved in water). In a natural stable environment, the dominant form of organic nitrogen in the soil is amino acids, therefore, from the point of view of “Soil Health”, the analysis of water-soluble organic nitrogen is a successful and informative method.
If we do not take into account the introduction of nitrogen fertilizers, then there are two main sources of nitrogen entering the soil: 1. as a product of the vital activity of soil biomass; 2. from organic matter. All this nitrogen can also be taken into account when considering the need for fertilizers. It is believed that from one to three percent of nitrogen enters the soil during mineralization from organic matter.
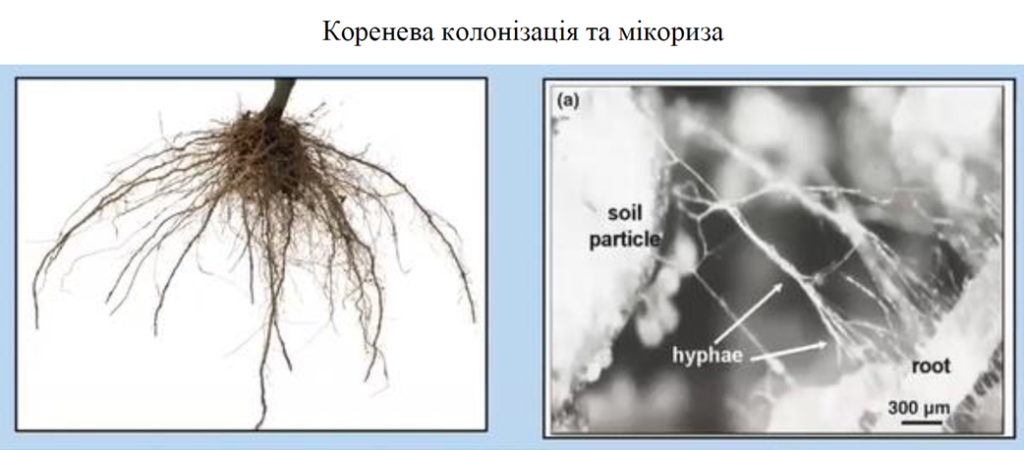
Standard agrochemical soil analysis uses a variety of different solutions (such as ammonium acetate, DTPA, Mehlich III, and others) that measure plant-available nutrients as well as total plant-absorbable minerals. To analyze the total amount of nutrients (most of them are unavailable forms that can be considered as a mineral reserve), the sample decomposition procedure (digest) is used; so let’s not confuse these two different chemical methods. But how do plants get these unavailable forms of nutrients? Answer: only thanks to microorganisms and mycorrhizal connection. All necessary mineral nutrients, which are critical for plant health, but are in unavailable form, can be obtained by plants only through the connection with mycorrhizae. Nature provides us with all these nutrients for free, but the most important thing is that we need to understand how these substances are released (become available to plants).
The percentage of bacterial colonization can be measured by microscopy and microscopic methods, which are very accurate but time-consuming and expensive. To identify this percentage of bacterial colonization, plant roots (as working material) are used, because this is where the exchange of nutrients takes place.
However, there are currently two new and very sensitive methods: “Soil respiration” and the PLFA test (Phospholipid Fatty Acids Test – analysis of phospholipid fatty acids).
Soil respiration provides information on the total number of microbial communities based on how much carbon dioxide is released from the soil. The soil sample is placed in a small glass jar, moistened with a small amount of water, closed with a lid and kept in an incubator for 24 hours in a warm environment. After 24 hours, the amount of carbon dioxide released from this soil is measured.

The PLFA test (Phospholipid Fatty Acids Test) is an analysis aimed at detecting the total microbial biomass thanks to special biomarkers that are specific for certain microbial groups. These biomarkers are quantified by gas chromatography and provide information on the following soil communities: bacteria (gram positive and gram negative), fungi, mycorrhizae, actinomycetes, nitrogen-fixing bacteria and protozoa.
ТI would like to say something about the stability of the soil aggregate. The stability of the soil aggregate is the property of the soil to maintain a single structure (due to microbial and plant secretions) together with minerals, organic matter, fungi, plant roots and everything present in the soil. It is also an indicator of how likely it is that the soil can break down into components in water or under the action of water. Stable aggregates are the end product of a healthy and productive soil that is able to retain water. It is known that forest soils have a higher proportion of stable aggregates compared to cultivated soil, because they are practically undisturbed. Physical destruction of the soil breaks these stable aggregates and disrupts the entire microbial community, their life and work. Soils with a high clay content should have a higher stable fraction compared to sandy soils. Aggregate stability is also a constituent parameter in the general assessment of the state of “soil health”.
This article is based on information courtesy of the laboratory Ward Laboratories, Inc.
Write to us
and we will find an opportunity
for cooperation